You Use The Combination Gas Law When These Variables Are Constant . The combined gas law is also often written as two. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. however, situations do arise where all three variables change. The exact value of k will depend on the moles of gas. It states the the ratio between the. but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured. the combined gas law combines the three gas laws: It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant. the equations describing these laws are special cases of the ideal gas law, pv = nrt, where p is the pressure of the gas, v is its. and k is a constant. The combined gas law expresses the relationship between the. What gas law would you use if the pressure, volume, and moles changed while the temperature remained constant?.
from www.chegg.com
It states the the ratio between the. but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured. The combined gas law is also often written as two. the combined gas law combines the three gas laws: What gas law would you use if the pressure, volume, and moles changed while the temperature remained constant?. however, situations do arise where all three variables change. The exact value of k will depend on the moles of gas. It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. the equations describing these laws are special cases of the ideal gas law, pv = nrt, where p is the pressure of the gas, v is its.
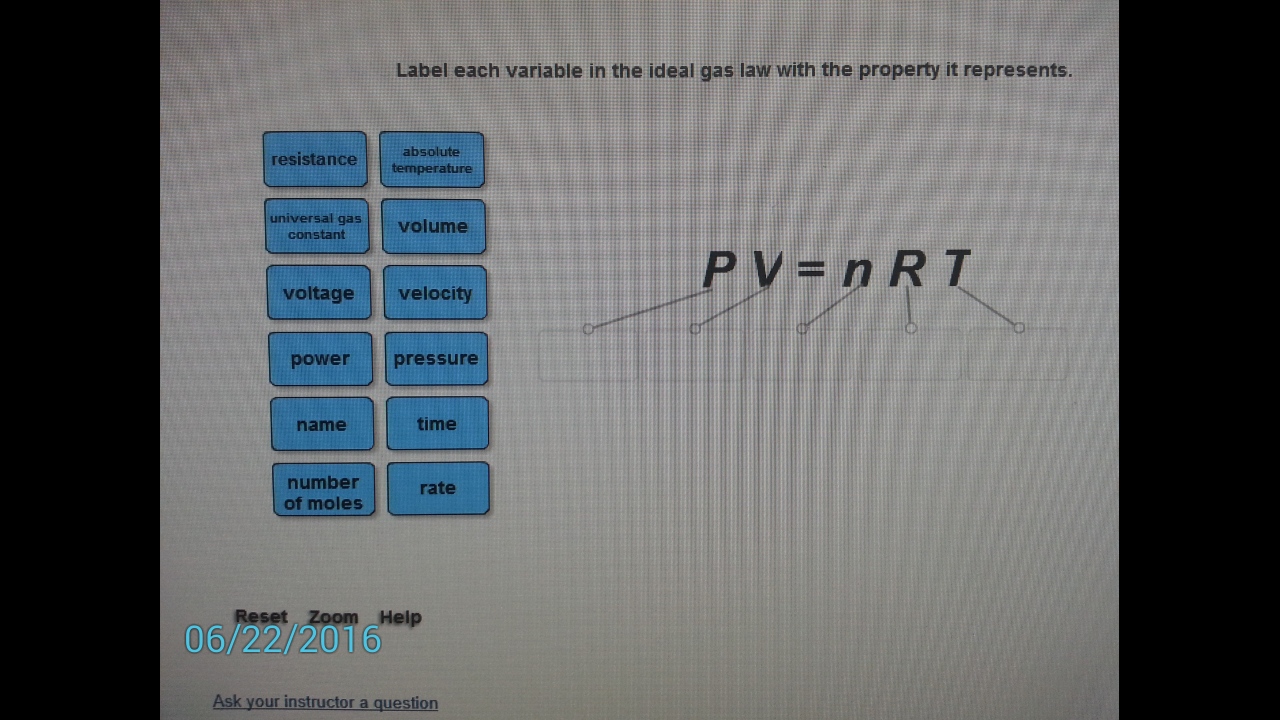
Solved Label each variable in the ideal gas law with the
You Use The Combination Gas Law When These Variables Are Constant The combined gas law expresses the relationship between the. The combined gas law expresses the relationship between the. however, situations do arise where all three variables change. but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. the equations describing these laws are special cases of the ideal gas law, pv = nrt, where p is the pressure of the gas, v is its. It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant. It states the the ratio between the. and k is a constant. the combined gas law combines the three gas laws: The exact value of k will depend on the moles of gas. What gas law would you use if the pressure, volume, and moles changed while the temperature remained constant?. The combined gas law is also often written as two.
From www.showme.com
ShowMe derivation of combined gas law You Use The Combination Gas Law When These Variables Are Constant The combined gas law expresses the relationship between the. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. and k is a constant. the combined gas law combines the three gas laws: What gas law would you use if the pressure, volume, and moles changed while. You Use The Combination Gas Law When These Variables Are Constant.
From sciencenotes.org
Ideal Gas Law Formula and Examples You Use The Combination Gas Law When These Variables Are Constant The combined gas law expresses the relationship between the. The combined gas law is also often written as two. It states the the ratio between the. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. the equations describing these laws are special cases of the ideal gas. You Use The Combination Gas Law When These Variables Are Constant.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal You Use The Combination Gas Law When These Variables Are Constant the combined gas law combines the three gas laws: The combined gas law expresses the relationship between the. the equations describing these laws are special cases of the ideal gas law, pv = nrt, where p is the pressure of the gas, v is its. It states the the ratio between the. but we can use each. You Use The Combination Gas Law When These Variables Are Constant.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net You Use The Combination Gas Law When These Variables Are Constant The combined gas law is also often written as two. but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured. The exact value of k will depend on the moles of gas. It states the the ratio between the. Web. You Use The Combination Gas Law When These Variables Are Constant.
From www.inspiritvr.com
Universal Gas Law Study Guide Inspirit Learning Inc You Use The Combination Gas Law When These Variables Are Constant but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. the combined gas law combines the three gas. You Use The Combination Gas Law When These Variables Are Constant.
From mungfali.com
Ideal Gas Law Chart You Use The Combination Gas Law When These Variables Are Constant the combined gas law combines the three gas laws: What gas law would you use if the pressure, volume, and moles changed while the temperature remained constant?. The combined gas law expresses the relationship between the. The combined gas law is also often written as two. however, situations do arise where all three variables change. the equations. You Use The Combination Gas Law When These Variables Are Constant.
From animalia-life.club
Combined Gas Law Worksheet Answers You Use The Combination Gas Law When These Variables Are Constant It states the the ratio between the. The combined gas law expresses the relationship between the. What gas law would you use if the pressure, volume, and moles changed while the temperature remained constant?. the equations describing these laws are special cases of the ideal gas law, pv = nrt, where p is the pressure of the gas, v. You Use The Combination Gas Law When These Variables Are Constant.
From korbin-well-orozco.blogspot.com
Formulas Used to Describe Gas Behavior You Use The Combination Gas Law When These Variables Are Constant and k is a constant. The exact value of k will depend on the moles of gas. but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured. It states the the ratio between the. The combined gas law is. You Use The Combination Gas Law When These Variables Are Constant.
From bceweb.org
Combined Gas Law Complete The Chart A Visual Reference of Charts You Use The Combination Gas Law When These Variables Are Constant The exact value of k will depend on the moles of gas. It states the the ratio between the. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. the combined gas law combines the three gas laws: What gas law would you use if the pressure, volume,. You Use The Combination Gas Law When These Variables Are Constant.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985 You Use The Combination Gas Law When These Variables Are Constant however, situations do arise where all three variables change. but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained. You Use The Combination Gas Law When These Variables Are Constant.
From einmalalle.blogspot.com
Ideal Gas Law R Values / Why are Gas Laws Important in Chemistry You Use The Combination Gas Law When These Variables Are Constant however, situations do arise where all three variables change. The combined gas law expresses the relationship between the. It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant. and k is a constant. The exact value of k will depend on the moles of. You Use The Combination Gas Law When These Variables Are Constant.
From www.numerade.com
SOLVEDThe ideal gas law relates four variables. An empirical gas law You Use The Combination Gas Law When These Variables Are Constant What gas law would you use if the pressure, volume, and moles changed while the temperature remained constant?. The combined gas law expresses the relationship between the. The combined gas law is also often written as two. It states the the ratio between the. the combined gas law combines the three gas laws: the equations describing these laws. You Use The Combination Gas Law When These Variables Are Constant.
From www.studocu.com
MM+Annotated+Gas+Law+Basics Gas Variables What are 4 variables of a You Use The Combination Gas Law When These Variables Are Constant It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant. The combined gas law is also often written as two. but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant,. You Use The Combination Gas Law When These Variables Are Constant.
From mmerevise.co.uk
The Ideal Gas Equation MME You Use The Combination Gas Law When These Variables Are Constant What gas law would you use if the pressure, volume, and moles changed while the temperature remained constant?. The exact value of k will depend on the moles of gas. but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured.. You Use The Combination Gas Law When These Variables Are Constant.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID You Use The Combination Gas Law When These Variables Are Constant and k is a constant. It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant. the equations describing these laws are special cases of the ideal gas law, pv = nrt, where p is the pressure of the gas, v is its. The combined. You Use The Combination Gas Law When These Variables Are Constant.
From chemistryguru.com.sg
Ideal Gas Law and Applications You Use The Combination Gas Law When These Variables Are Constant What gas law would you use if the pressure, volume, and moles changed while the temperature remained constant?. The combined gas law is also often written as two. the equations describing these laws are special cases of the ideal gas law, pv = nrt, where p is the pressure of the gas, v is its. It states that the. You Use The Combination Gas Law When These Variables Are Constant.
From www.chegg.com
Solved Label each variable in the ideal gas law with the You Use The Combination Gas Law When These Variables Are Constant however, situations do arise where all three variables change. but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured. the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained. You Use The Combination Gas Law When These Variables Are Constant.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID You Use The Combination Gas Law When These Variables Are Constant but we can use each of the empirical gas laws as a special case of the ideal gas law, defined by which variables are constant, and which are measured. It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant. the equations describing these laws. You Use The Combination Gas Law When These Variables Are Constant.